FOOD and Drug Administration has warned of unregistered oxytocin tablets which have no guarantee of quality and how much it effects or harmful substances.
It has carried out regular checks on the pharmaceutical market and low-quality unregistered drugs are found selling in the market, so the public should be cautious and do not use them, FDA said in a statement on 17 February.
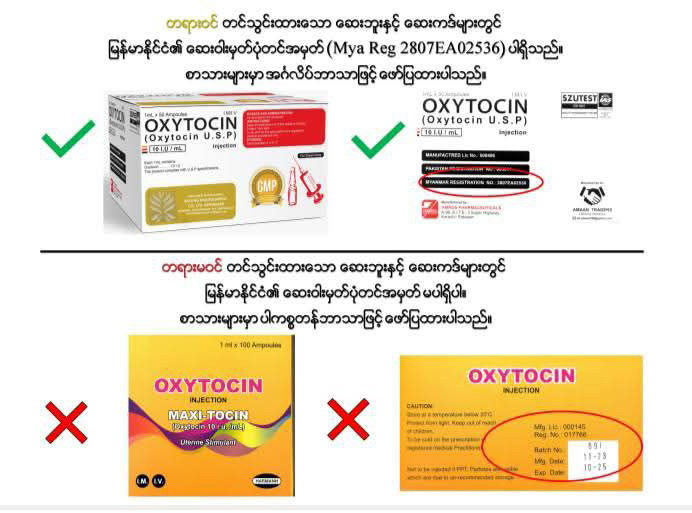
FDA has made public drug photos to help people to distinguish what is registered and what are unregistered and the statement stipulated four oxytocin brands which are unofficially imported and written in Pakistan, said the statement.
It said oxytocin USP is an officially registered drug featuring a Myanmar registration number and written in English. — MT/ZS